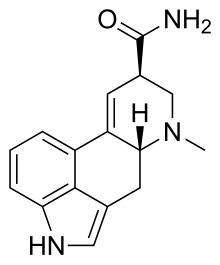
Ergine
This article needs additional citations for verification. (July 2007) |
 | |
 | |
| Clinical data | |
|---|---|
| Other names | LSA; d-Lysergic acid amide; d-Lysergamide; Ergine; LA-111 |
| Pregnancy category |
|
| Routes of administration | Oral, intramuscular injection |
| ATC code |
|
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.006.841 |
| Chemical and physical data | |
| Formula | C16H17N3O |
| Molar mass | 267.332 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 135 °C (275 °F) Decomposes[4] |
| |
| |
| (verify) | |
Ergine, also known as d-lysergic acid amide (LSA) and d-lysergamide, is an ergoline alkaloid that occurs in various species of vines of the Convolvulaceae and some species of fungi. The psychedelic properties in the seeds of ololiuhqui, Hawaiian baby woodrose and morning glories have been linked to ergine and/or isoergine, its epimer, as it is an alkaloid present in the seeds.[5][6][7]
Occurrence in nature
[edit]Ergine is not a biosynthetic endpoint itself, but rather a hydrolysis product of lysergic acid hydroxyethylamide (LAH), lysergic acid hydroxymethylethylamide (ergonovine), and ergopeptines or their ergopeptam precursors.[8][9][10][11][12]
Lysergic acid hydroxyethylamide is very vulnerable to this hydrolysis,[13][14] and many analyses of ergoline-containing fungi show little to no LAH and substantial amounts of ergine.
Ergine, LAH, and ergonovine are natural ergoamides. An ergine analog, 8-hydroxyergine, has also been found in natural products in two studies.[15][16] Methylergonovine and methylmethylergonovine (methysergide) have also been found in a natural product in only one study;[17] these are documented as semisynthetic chemicals, so the findings need to be repeated for certainty. The aforementioned chemicals are the only natural ergoamides.
LAH & ergine are predominant in Claviceps paspali,[18][19][20] but are only found in trace amounts in the more well-known Claviceps purpurea[21][22] (both are ergot-spreading fungi). The major products of C. purpurea are ergopeptines, but C. paspali does not generate ergopeptines.[23] Ergonovine is the only ergoamide in C. purpurea in substantial amounts.[24]
LAH & ergine are also found in the related fungi, Periglandula, which are permanently connected with Ipomoea tricolor, Ipomoea corymbosa, Argyreia nervosa (“morning glory”, coaxihuitl, Hawaiian baby woodrose), and an estimated over 440 other Convolvulaceae[25] (ergolines have been identified in 42 of these plants and not all of them contain ergine[26]). Ipomoea tricolor contains 1/6th the amount of ergonovine of ergine.[27]
Other fungi that have been found to contain LAH and/or ergine
All of these fungi are related to Claviceps fungi.
Unidentified Acremonium species that infects sleepy grass (C. purpurea also infects sleepy grass[28]).[29]
Unidentified Acremonium species that infects drunken horse grass[30]
Acremonium coenophialum (infects Festuca arundinacea)[31]
Epichloë gansuensis var. inebriens (infects drunken horse grass)[32]
Metarhizium brunneum[33]
Metarhizium acridum[33]
Metarhizium anisopliae[33]
Metarhizium flavoviride[33]
Metarhizium robertsii[33]
Aspergillus leporis,[34]
Aspergillus homomorphus[34]
Aspergillus hancockii[34]
[Aspergillus is considered to be a more distant relative of Claviceps.]
Other fungi that possibly contain ergine (i.e. they have been found to contain ergonovine and/or ergopeptines):
Claviceps hirtella[35]
Neotyphodium lolii[36]
Unidentified Epichlöe and Neotyphodium (asexual forms of Epichlöe) species[37]
Aspergillus fumigata,[38] Aspergillus flavus[38]
Botritis fabae[38]
Curvularia lunata[38]
Geotrichum candidum[38]
Balansia cyperi,[38] Balansia claviceps,[38] Balansia epichloë[38]
Epichloë amarillans[39]
Epichloë cabralii (H)[40]
Epichloë canadensis (H)[41][42]
Epichloë coenophiala (H)[41][43][44][45]
Epichloë festucae[39]
Epichloë festucae var. lolii[46][47]
Epichloë festucae var. lolii x E. typhina (H)[41][48]
Epichloë inebriens[39]
Epichloë glyceriae[39]
Epichloë mollis[41]
Epichloë typhina[38]
Epichloë typhina ssp. poae[39][40]
Epichloë typhina ssp. clarkii[49]
Epichloë sp. AroTG-2(H)[50]
Epichloë sp. FaTG-2(H)[41][43][45][51][52]
Epichloë sp. FaTG-4(H)[41][45]
Hypomyces aurantius[38]
Sepedonium sp.[38]
Cunnigbamella blakesleana[38]
Mucor biemalis[38]
Rhizopus nigricans[38]
History
[edit]Ololiuhqui was used by South American healers in shamanic healing ceremonies.[53] Similarly, ingestion of morning glory seeds by Mazatec tribes to "commune with their gods" was reported by Richard Schultes in 1941 and is still practiced today.[54][53]
Additional reports of the use of ergine were made by Don Thomes MacDougall. He reported that the seeds of Ipomoea violacea were used as sacraments by certain Zapotecs, sometimes in conjunction with the seeds of Rivea corymbosa, another species which has a similar chemical composition, with lysergol instead of ergometrine.[7]
Ergine was assayed for human activity by Albert Hofmann in self-trials in 1947, well before it was known to be a natural compound. Intramuscular administration of a 500 microgram dose led to a tired, dreamy state, with an inability to maintain clear thoughts. After a short period of sleep the effects were gone, and normal baseline was recovered within five hours.[6]
In 1956, the Central Intelligence Agency conducted research on the psychedelic properties of the ergine in the seeds of Rivea corymbosa, as Subproject 22 of MKULTRA.[55]
In 1959, Hofmann was the first to isolate chemically pure ergine from the seeds of Turbina corymbosa, determining that it, and other alkaloids, were acting as the main active components in the seeds.[7] Twenty years prior to its isolation, ergine was first chemically defined by English chemists S. Smith and G. M. Timmis as the cleavage product of ergot alkaloids. Additionally, Guarin and Youngkin reportedly isolated the crude alkaloid in 1964 from morning glory seeds.[56]
Ingestion
[edit]Like other psychedelics, ergine is not considered to be addictive. Additionally, there are no known deaths directly associated with pharmacological effects of ergine consumption. All associated deaths are due to indirect causes, such as self-harm, impaired judgement, and adverse drug interactions. One known case involved a suicide that was reported in 1964 after ingestion of morning glory seeds.[57] Another instance is a death due to falling off of a building after ingestion of Hawaiian baby woodrose seeds and alcohol.[58]
Physiological effects
[edit]While its physiological effects vary from person to person, the following symptoms have been attributed to the consumption of ergine or ergine containing seeds:[7][53][59]
- Sedation
- Visual and auditory hallucinations
- Euphoria
- Loss of motor control
- Nausea
- Vasoconstriction
- Delusion
- Anxiety
- Paranoia
- Irregular heartbeat[60]
- Sexual arousal[61]
- Tachycardia[62]
- Mydriasis[62]
- Hypertonia[62]
- Respiratory disturbances[62]
- Cramps[62]
One study found that 2 of 4 human subjects experienced cardiovascular dysregulation and the study had to be halted, concluding that the ingestion of seeds containing ergine was less safe then commonly believed. Importantly this may have been a product of other substances within the seeds. The same study also observed that reactions were highly differing in type and intensity between different subjects.[60] Another study in mice found that the drug had aphrodisiac properties, inducing increased sexual behavior.[61]
A study gave mice 3000 mg/kg with no lethal effects.[citation needed]
Psychedelic component
[edit]Ergine is thought to be a serotonergic psychedelic and its psychedelic effects are thought to be due to it being a partial agonist of the 5-HT2A receptor. Though, the reason as to why this may be hallucinogenic remains elusive.
The idea that ergine is the main psychedelic component in ergine containing seeds (morning glory, Hawaiian baby woodrose) is well debated, as the effects of isolated synthetic ergine are reported to be only mildly psychedelic.[63][59] Thus, the overall psychedelic experience after consumption of such seeds has been proposed to be due to a mixture of ergoline alkaloids.
Pharmacology
[edit]Pharmacodynamics
[edit]| Receptor | Affinity (Ki [nM]) | |
|---|---|---|
| LSA | LSD | |
| 5-HT1A | 10 | 2.5 |
| 5-HT2 | 28 | 0.87 |
| D1 | 832 | 87 |
| D2L | 891 | 155 |
| D2S | 145 | 25 |
| D3 | 437 | 65 |
| D4.4 | 141 | 30 |
| α1 | 912 | 60 |
| α2 | 62 | 1.0 |
| Notes: 5-HT1A and D1 are for pig receptors.[64] | ||
Ergine interacts with serotonin, dopamine, and adrenergic receptors similarly to but with lower affinity than lysergic acid diethylamide (LSD).[64][65] The psychedelic effects of ergine can be attributed to activation of serotonin 5-HT2A receptors.[66]
Chemistry
[edit]Biosynthesis
[edit]
The biosynthetic pathway to ergine starts like most other ergoline alkaloid- with the formation of the ergoline scaffold. This synthesis starts with the prenylation of L-tryptophan in an SN1 fashion with dimethylallyl diphosphate (DMAPP) as the prenyl donor and catalyzed by prenyltransferase 4-dimethylallyltryptophan synthase (DMATS), to form 4-L-dimethylallyltryptophan (4-L-DMAT). The DMAPP is derived from mevalonic acid. A three strep mechanism is proposed to form 4-L-DMAT: the formation of an allylic carbocation, a nucleophilic attack of the indole nucleus to the cation, followed by deprotonation to restore aromaticity and to generate 4-L-DMAT.[67] 4-Dimethylallyltyptophan N-methyltransferase (EasF) catalyzes the N-methylation of 4-L-DMAT at the amino of the tryptophan backbone, using S-Adenosyl methionine (SAM) as the methyl source, to form 4-dimethylallyl-L-abrine (4-DMA-L-abrine).[67] The conversion of 4-DMA-L-abrine to chanoclavine-I is thought to occur through a decarboxylation and two oxidation steps, catalyzed by the FAD dependent oxidoreductase, EasE, and the catalase, EasC. The chanoclavine intermediate is then oxidized to chanoclavine-l-aldehyde, catalyzed by the short-chain dehydrogenase/reductase (SDR), EasD.[67][68]

From here, the biosynthesis diverges and the products formed are plant and fungus-specific. The biosynthesis of ergine in Claviceps purpurea will be exemplified, in which agroclavine is produced following the formation of chanoclavine-l-aldehyde, catalyzed by EasA through a keto-enol tautomerization to facilitate rotation about the C-C bond, followed by tautomerization back to the aldehyde and condensation with the proximal secondary amine to form an iminium species, which is subsequently reduced to the tertiary amine and yielding argoclavine.[67][68] Cytochrome P450 monooxygenases (CYP450) are then thought to catalyze the formation of elymoclavine from argoclavine via a 2 electron oxidation. This is further converted to paspalic acid via a 4 electron oxidation, catalyzed by cloA, a CYP450 monooxygenase. Paspalic acid then undergoes isomerization of the C-C double bond in conjugation with the acid to form D-lysergic acid.[67] While the specifics of the formation of ergine from D-lysergic acid are not known, it is proposed to occur through a nonribosomal peptide synthase (NRPS) with two enzymes primarily involve: D-lysergyl peptide synthase (LPS) 1 and 2.[67][68]

Legal status
[edit]The legality of consuming, cultivating, and possessing ergine varies depending on the country.
There are no laws against possession of ergine-containing seeds in the United States. However, possession of the pure compound without a prescription or a DEA license would be prosecuted, as ergine, under the name "lysergic acid amide", is listed under Schedule III of the Controlled Substances Act.[69] Similarly, ergine is considered a Class A substance in the United Kingdom, categorized as a precursor to LSD.
In most Australian states, the consumption of ergine containing materials is prohibited under state legislation.
In Canada, ergine is not illegal to possess as it is not listed under Canada's Controlled Drugs and Substances Act, though it is likely illegal to sell for human consumption.[70]
In New Zealand, ergine is a controlled drug, however the plants and seeds of the morning glory species are legal to possess, cultivate, buy, and distribute.
See also
[edit]- Argyreia nervosa
- List of entheogenic/hallucinogenic species
- List of psychoactive plants
- Tlitliltzin (Ipomoea violacea)
References
[edit]- ^ Erowid Morning Glory Basics, Erowid.org, retrieved 2012-02-03
- ^ Anvisa (2023-07-24). "RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-07-25). Archived from the original on 2023-08-27. Retrieved 2023-08-27.
- ^ "Arrêté du 20 mai 2021 modifiant l'arrêté du 22 février 1990 fixant la liste des substances classées comme stupéfiants". www.legifrance.gouv.fr (in French). 20 May 2021.
- ^ Smith S, Timmis GM (1932). "98. The alkaloids of ergot. Part III. Ergine, a new base obtained by the degradation of ergotoxine and ergotinine". Journal of the Chemical Society (Resumed): 763. doi:10.1039/jr9320000763.
- ^ Perrine DM (2000). "Mixing the Kykeon" (PDF). ELEUSIS: Journal of Psychoactive Plants and Compounds. New Series 4: 9. Archived from the original (PDF) on 2019-07-20. Retrieved 2008-05-05.
- ^ a b Alexander Shulgin, "#26. LSD-25", TiHKAL, Erowid.org, retrieved 2012-02-03
- ^ a b c d Hofmann A (2009). LSD My Problem Child: Reflections on Sacred Drugs, Mysticism, and Science (4th ed.). MAPS.org. ISBN 978-0979862229.
- ^ Flieger M, Sedmera P, Vokoun J, Ricicova A, Rehacek Z (1982-02-19). "Separation of four isomers of lysergic acid α-hydroxyethylamide by liquid chromatography and their spectroscopic identification". Journal of Chromatography A. 236 (2): 441–452. doi:10.1016/S0021-9673(00)84895-5. ISSN 0021-9673.
- ^ Ramstad E (1968). "Chemistry of alkaloid formation in ergot". Lloydia. 31: 327–341.
- ^ Kleinerová E, Kybal J (September 1973). "Ergot alkaloids: IV. Contribution to the biosynthesis of lysergic acid amides". Folia Microbiologica. 18 (5): 390–392. doi:10.1007/BF02875934. ISSN 0015-5632. PMID 4757982.
- ^ Panaccione DG, Tapper BA, Lane GA, Davies E, Fraser K (2003-10-01). "Biochemical Outcome of Blocking the Ergot Alkaloid Pathway of a Grass Endophyte". Journal of Agricultural and Food Chemistry. 51 (22): 6429–6437. doi:10.1021/jf0346859. ISSN 0021-8561. PMID 14558758.
- ^ Panaccione, D.G. Ergot alkaloids. In The Mycota, Industrial Applications, 2nd ed.; Hofrichter, M., Ed.; Springer-Verlag: Berlin-Heidelburg, Germany, 2010; Volume 10, pp. 195–214.
- ^ Shulgin A (1976). "4. Psychotomimetic Agents". In Maxwell G (ed.). Psychopharmacological agents. Medicinal Chemistry. Vol. 4. New York: Academic Press. pp. 59–00. ISBN 978-0-12-290559-9.
“The monohydroxyethylamides of each of these two materials are also principal components of the various morning glorys; viz., lysergic acid-α-hydroxyethylamide (VIII) and isolysergic acid-α-hydroxyethylamide (XI). These two carbinolamides are the principal ergot products of culture medium synthesis from Claviceps paspali, from which they can be prepared in concentrations of grams per liter of culture medium. These compounds, although well documented as components in the Convolvulaceae, are possibly lost in several of the analyses of alkaloid composition. They are extremely unstable, and are very readily degraded into acetaldehyde and the corresponding amide, ergine or isoergine. In these instances their presence will be measured only by the elevated levels of the derived amides.” B. Lysergamides from the Convolvulaceae spp. 1. Botany and Chemistry, pages 71 & 72 - ^ Schultes RE, Hofmann A (1973). The Botany and Chemistry of Hallucinogens. Springfield, IL: Charles Thomas. ISBN 9780398064167.
“Later, it was found that ergine and isoergine were present in the seeds to some extent in the form of lysergic acid N-(1-hydroxyethyl) amide and isolysergic acid N-(1-hydroxyethyl) amide, respectively, and that, during the isolation procedure, they easily hydrolize to ergine and isoergine, respectively, and acetaldehyde.” 4. Plants of Hallucinogenic Use / Convolvulaceae, p. 246 - ^ Flieger M, Linhartová R, Sedmera P, Zima J, Sajdl P, Stuchlík J, et al. (September 1, 1989). "New Alkaloids of Claviceps paspali". Journal of Natural Products. 52 (5): 1003–1007. doi:10.1021/np50065a014. ISSN 0163-3864.
- ^ Petroski RJ, Powell RG, Clay K (March–April 1992). "Alkaloids of Stipa robusta (sleepygrass) infected with an Acremonium endophyte". Natural Toxins. 1 (2): 84–88. doi:10.1002/nt.2620010205. ISSN 1056-9014. PMID 1344912.
“8-Hydroxylysergic acid amide was isolated with difficulty as it was present as only a minor alkaloid in endophyte-infected sleepygrass (0.3 pg/g dry wt).” Results and Discussion, p. 87 - ^ Paulke A, Kremer C, Wunder C, Wurglics M, Schubert-Zsilavecz M, Toennes SW (March 10, 2015). "Studies on the alkaloid composition of the Hawaiian Baby Woodrose Argyreia nervosa, a common legal high". Forensic Science International. 249: 281–293. doi:10.1016/j.forsciint.2015.02.011. PMID 25747328.
“On the other hand, methylergometrine, methysergide, and lysergylalanine were detected, which have not yet been reported as compounds of Argyreia nervosa seeds.” 3. Results and Discussion, p. 283 - ^ Arcamone F, Bonino C, Chain EB, Ferretti A, Pennella P, Tonolo A, et al. (1960). "Production of Lysergic Acid Derivatives by a Strain of Claviceps paspali Stevens and Hall in Submerged Culture". Nature. 187 (4733): 238–239. doi:10.1038/187238a0. ISSN 0028-0836. PMID 13794048.
- ^ Castagnoli N, Corbett K, Chain EB, Thomas R (1970-04-01). "Biosynthesis of N -(α-hydroxyethyl)-lysergamide, a metabolite of Claviceps paspali Stevens & Hall". Biochemical Journal. 117 (3): 451–455. doi:10.1042/bj1170451. ISSN 0306-3283. PMC 1178946. PMID 5419742.
- ^ Basmadjian G, Floss HG, Gröger D, Erge D (1969). "Biosynthesis of ergot alkaloids. Lysergylalanine as precursor of amide-type alkaloids". J. Chem. Soc. D (8): 418–419. doi:10.1039/C29690000418. ISSN 0577-6171.
- ^ Schultes R (1973). The Botany and Chemistry of Hallucinogens. Springfield, IL: Charles Thomas. ISBN 9780398064167.
“Whereas ergine, lysergic acid hydroxyethylamide, and lysergyl L-valine methylester occur in ergot of rye only in trace amounts, ergonovine (synonyms ergometrine, ergobasin), which is the specific oxytocic factor of a ergot, is often found in remarkable quantities. In contrast, ergine and hydroxyethylamide of lysergic acid are the main constituents of certain ergot growing on wild grasses, e.g. Paspalum distichum.” 4. Plants of Hallucinogenic Use / The Fungi, p. 37 - ^ Wasson RG, Hofmann A, Ruck CA, Webster P (November 25, 2008). Forte R (ed.). The Road to Eleusis: Unveiling the Secret of the Mysteries (30th Anniversary ed.). Berkeley, Calif.: North Atlantic Books. ISBN 978-1-55643-752-6.
“We analyzed ergot of wheat and ergot of barley in our laboratory and they were found to contain basically the same alkaloids as ergot of rye, viz. alkaloids of the ergotamine and ergotoxine group, ergonovine, and sometimes also traces of lysergic acid amide. As I said before, ergonovine and lysergic acid amide, both psychoactive, are soluble in water whereas the other alkaloids are not.” 2. A Challenging Question and My Answer, p. 42 (Hofmann) - ^ Panaccione, D.G. Ergot alkaloids. In The Mycota, Industrial Applications, 2nd ed.; Hofrichter, M., Ed.; Springer-Verlag: Berlin-Heidelburg, Germany, 2010; Volume 10, pp. 195–214
“C. paspali produces simple amides of lysergic acid (ergonovine, lysergic acid α-hydroxyethylamide, ergine) but not ergopeptines. Such a profile could be explained by a battery of ergot alkaloid biosynthetic genes similar to those found in C. purpurea but lacking the peptide synthetase LPS1 encoded by lpsA. This explanation is hypothetical because ergot pathway genes have not yet been analyzed in C. paspali.” 2. Typical Terminal Branch in Clavicipitaceous Ergot Alkaloid Producers, p. 205 - ^ Wasson RG, Hofmann A, Ruck CA, Webster P (November 25, 2008). Forte R (ed.). The Road to Eleusis: Unveiling the Secret of the Mysteries (30th Anniversary ed.). Berkeley, Calif.: North Atlantic Books. ISBN 978-1-55643-752-6.
“Samples of ergot grown on L. temulentum and L . perenne collected in Germany, France, and Switzerland showed large variation in their alkaloidal composition. Some contained substantial amounts of ergonovine together with alkaloids of the ergotamine and ergotoxine group.2”
“2. Kobel, H., Sandoz Research Laboratories, Basel. Private communication.”
2. A Challenging Question and My Answer, p. 44 (Hofmann) - ^ Leistner E, Steiner U (February 3, 2018), Anke T, Schüffler A (eds.), "The Genus Periglandula and Its Symbiotum with Morning Glory Plants (Convolvulaceae)", Physiology and Genetics, Cham: Springer International Publishing, pp. 131–147, doi:10.1007/978-3-319-71740-1_5, ISBN 978-3-319-71739-5, retrieved 2024-11-21
- ^ Eich E (January 12, 2008). "4.2 Ergolines". Solanaceae and convolvulaceae - secondary metabolites: biosynthesis, chemotaxonomy, biological and economic significance: a handbook. Berlin, Heidelberg: Springer-Verlag. doi:10.1007/978-3-540-74541-9. ISBN 978-3-540-74540-2. OCLC 195613136.
Table 4.1 Unambiguously ergoline-positive Ipomoea species (pages 225-227)
Table 4.4 Unambiguously ergoline-positive Argyreia species (p. 236)
Table 4.5 Unambiguously ergoline-positive Stictocardia and Turbina species (p. 238) - ^ Nowak J, Woźniakiewicz M, Klepacki P, Sowa A, Kościelniak P (February 14, 2016). "Identification and determination of ergot alkaloids in Morning Glory cultivars". Analytical and Bioanalytical Chemistry. 408 (12): 3093–3102. doi:10.1007/s00216-016-9322-5. ISSN 1618-2642. PMC 4830885. PMID 26873205.
See table 3
Values for “LSH”, “Lyzergol/isobars”, penniclavine, and chanoclavine can be obtained by dividing the concentration values of ergine or ergometrine by their relative abundance values and multiplying that number by the relative abundance value of the specified chemical. - ^ Alderman SC, Halse RR, White JF (January 2004). "A Reevaluation of the Host Range and Geographical Distribution of Claviceps Species in the United States". Plant Disease. 88 (1): 63–81. doi:10.1094/PDIS.2004.88.1.63. ISSN 0191-2917. PMID 30812458.
- ^ Petroski RJ, Powell RG, Clay K (March–April 1992). "Alkaloids of Stipa robusta (sleepygrass) infected with an Acremonium endophyte". Natural Toxins. 1 (2): 84–88. doi:10.1002/nt.2620010205. ISSN 1056-9014. PMID 1344912.
- ^ Miles CO, Lane GA, di Menna ME, Garthwaite I, Piper EL, Ball OJ, et al. (1996-05-16). "High Levels of Ergonovine and Lysergic Acid Amide in Toxic Achnatherum inebrians Accompany Infection by an Acremonium -like Endophytic Fungus". Journal of Agricultural and Food Chemistry. 44 (5): 1285–1290. doi:10.1021/jf950410k. ISSN 0021-8561.
- ^ Hedin PA, ed. (1991-01-09). "Preparative Separation of Complex Alkaloid Mixture by High-Speed Countercurrent Chromatography". Naturally Occurring Pest Bioregulators. ACS Symposium Series. Vol. 449. Washington, DC: American Chemical Society. pp. 426–434. doi:10.1021/bk-1991-0449.ch031. ISBN 978-0-8412-1897-0.
- ^ Chen L, Li X, Li C, Swoboda GA, Young CA, Sugawara K, et al. (July 2015). "Two distinct Epichloë species symbiotic with Achnatherum inebrians , drunken horse grass". Mycologia. 107 (4): 863–873. doi:10.3852/15-019. ISSN 0027-5514. PMID 25911697. “… the [Epichloë gansuensis var. inebriens] isolates produce neurotropic lysergic acid amides …” (Abstract)
- ^ a b c d e Leadmon CE, Sampson JK, Maust MD, Macias AM, Rehner SA, Kasson MT, et al. (2020-07-02). Alexandre G (ed.). "Several Metarhizium Species Produce Ergot Alkaloids in a Condition-Specific Manner". Applied and Environmental Microbiology. 86 (14). doi:10.1128/AEM.00373-20. ISSN 0099-2240. PMC 7357478. PMID 32385081.
- ^ a b c Jones AM, Steen CR, Panaccione DG (2021-11-24). Atomi H (ed.). "Independent Evolution of a Lysergic Acid Amide in Aspergillus Species". Applied and Environmental Microbiology. 87 (24): e0180121. doi:10.1128/AEM.01801-21. ISSN 0099-2240. PMC 8612279. PMID 34586904.
- ^ Lorenz N, Haarmann T, Pažoutová S, Jung M, Tudzynski P (2009-10-01). "The ergot alkaloid gene cluster: Functional analyses and evolutionary aspects". Phytochemistry. Evolution of Metabolic Diversity. 70 (15–16): 1822–1832. doi:10.1016/j.phytochem.2009.05.023. PMID 19695648.
- ^ Fleetwood DJ, Scott B, Lane GA, Tanaka A, Johnson RD (2007-04-15). "A Complex Ergovaline Gene Cluster in Epichloë Endophytes of Grasses". Applied and Environmental Microbiology. 73 (8): 2571–2579. doi:10.1128/AEM.00257-07. ISSN 0099-2240. PMC 1855613. PMID 17308187.
- ^ Schardl CL, Leuchtmann A, Spiering MJ (2004-06-02). "Symbioses of Grasses with Seedborne Fungal Endophytes". Annual Review of Plant Biology. 55 (1): 315–340. doi:10.1146/annurev.arplant.55.031903.141735. ISSN 1543-5008. PMID 15377223.
- ^ a b c d e f g h i j k l m n Kozlovsky A (2006). "18. Producers of ergot alkaloids out of Claviceps genus". In Křen V, Cvak L (eds.). Ergot: The Genus Claviceps. Medicinal and aromatic plants - industrial profiles. London: Harwood Academic Publishers. ISBN 978-90-5702-375-0.
- ^ a b c d e Schardl CL, Young CA, Hesse U, Amyotte SG, Andreeva K, Calie PJ, et al. (2013-02-28). Heitman J (ed.). "Plant-Symbiotic Fungi as Chemical Engineers: Multi-Genome Analysis of the Clavicipitaceae Reveals Dynamics of Alkaloid Loci". PLOS Genetics. 9 (2): e1003323. doi:10.1371/journal.pgen.1003323. ISSN 1553-7404. PMC 3585121. PMID 23468653.
- ^ a b Charlton ND, Craven KD, Afkhami ME, Hall BA, Ghimire SR, Young CA (2014-10-01). "Interspecific hybridization and bioactive alkaloid variation increases diversity in endophytic Epichloë species of Bromus laevipes". FEMS Microbiology Ecology. 90 (1): 276–289. doi:10.1111/1574-6941.12393. PMID 25065688.
- ^ a b c d e f Schardl C, Young C, Pan J, Florea S, Takach J, Panaccione D, et al. (June 6, 2013). "Currencies of Mutualisms: Sources of Alkaloid Genes in Vertically Transmitted Epichloae". Toxins. 5 (6): 1064–1088. doi:10.3390/toxins5061064. ISSN 2072-6651. PMC 3717770. PMID 23744053.
- ^ Charlton ND, Craven KD, Mittal S, Hopkins AA, Young CA (Sep–Oct 2012). "Epichloe canadensis, a new interspecific epichloid hybrid symbiotic with Canada wildrye (Elymus canadensis)". Mycologia. 104 (5): 1187–1199. doi:10.3852/11-403. ISSN 0027-5514. PMID 22675049.
- ^ a b Takach JE, Mittal S, Swoboda GA, Bright SK, Trammell MA, Hopkins AA, et al. (2012-08-15). "Genotypic and Chemotypic Diversity of Neotyphodium Endophytes in Tall Fescue from Greece". Applied and Environmental Microbiology. 78 (16): 5501–5510. doi:10.1128/AEM.01084-12. ISSN 0099-2240. PMC 3406137. PMID 22660705.
- ^ Hanigan MH, Ricketts WA (June 1993). "Extracellular glutathione is a source of cysteine for cells that express gamma-glutamyl transpeptidase". Biochemistry. 32 (24): 6302–6. doi:10.1021/bi00075a026. PMID 8099811.
- ^ a b c Young CA, Charlton ND, Takach JE, Swoboda GA, Trammell MA, Huhman DV, et al. (2014-11-04). "Characterization of Epichloë coenophiala within the US: are all tall fescue endophytes created equal?". Frontiers in Chemistry. 2: 95. doi:10.3389/fchem.2014.00095. ISSN 2296-2646. PMC 4219521. PMID 25408942.
- ^ Fleetwood DJ, Scott B, Lane GA, Tanaka A, Johnson RD (2007-04-15). "A Complex Ergovaline Gene Cluster in Epichloë Endophytes of Grasses". Applied and Environmental Microbiology. 73 (8): 2571–2579. doi:10.1128/AEM.00257-07. ISSN 0099-2240. PMC 1855613. PMID 17308187.
- ^ Fleetwood DJ, Khan AK, Johnson RD, Young CA, Mittal S, Wrenn RE, et al. (2011-01-01). "Abundant Degenerate Miniature Inverted-Repeat Transposable Elements in Genomes of Epichloid Fungal Endophytes of Grasses". Genome Biology and Evolution. 3: 1253–1264. doi:10.1093/gbe/evr098. ISSN 1759-6653. PMC 3227409. PMID 21948396.
- ^ Panaccione DG, Johnson RD, Wang J, Young CA, Damrongkool P, Scott B, et al. (2001-10-23). "Elimination of ergovaline from a grass– Neotyphodium endophyte symbiosis by genetic modification of the endophyte". Proceedings of the National Academy of Sciences. 98 (22): 12820–12825. doi:10.1073/pnas.221198698. ISSN 0027-8424. PMC 60137. PMID 11592979.
- ^ Young C, Schardl C, Panaccione D, Florea S, Takach J, Charlton N, et al. (2015-04-16). "Genetics, Genomics and Evolution of Ergot Alkaloid Diversity". Toxins. 7 (4): 1273–1302. doi:10.3390/toxins7041273. ISSN 2072-6651. PMC 4417967. PMID 25875294. See table 3 on p. 1290.
- ^ Shymanovich T, Saari S, Lovin ME, Jarmusch AK, Jarmusch SA, Musso AM, et al. (2014-12-11). "Alkaloid Variation Among Epichloid Endophytes of Sleepygrass (Achnatherum robustum) and Consequences for Resistance to Insect Herbivores". Journal of Chemical Ecology. 41 (1): 93–104. doi:10.1007/s10886-014-0534-x. ISSN 0098-0331. PMID 25501262.
- ^ Christensen M, Leuchtmann A, Rowan D, Tapper B (1993-09-01). "Taxonomy of Acremonium endophytes of tall fescue (Festuca arundinacea), meadow fescue (F. pratensis) and perennial ryegrass (Lolium perenne)". Mycological Research. 97 (9): 1083–1092. doi:10.1016/S0953-7562(09)80509-1.
- ^ Panaccione DG, Johnson RD, Wang J, Young CA, Damrongkool P, Scott B, et al. (2001-10-23). "Elimination of ergovaline from a grass– Neotyphodium endophyte symbiosis by genetic modification of the endophyte". Proceedings of the National Academy of Sciences. 98 (22): 12820–12825. doi:10.1073/pnas.221198698. ISSN 0027-8424. PMC 60137. PMID 11592979.
- ^ Schultes RE (1941). A Contribution to Our Knowledge of Rivea Corymbosa: The Narcotic Ololinqui of the Aztecs (1st ed.). Botanical Museum of Harvard University.
- ^ "PROJECT MKULTRA, SUBPROJECT 22 (W/ATTACHMENTS)". Central Intelligence Agency.
- ^ Der Marderosian AH, Guarino AM, De Feo JJ, Youngken Jr HW (1964). "A Uterine Stimulant Effect of Extracts of Morning Glory Seeds". Pschedelic Review: 317–323.
- ^ Cohen S (April 1964). "Suicide Following Morning Glory Seed Ingestion". The American Journal of Psychiatry. 120 (1): 1024–1025. doi:10.1176/ajp.120.10.1024. PMID 14138842.
- ^ Klinke HB, Müller IB, Steffenrud S, Dahl-Sørensen R (April 2010). "Two cases of lysergamide intoxication by ingestion of seeds from Hawaiian Baby Woodrose". Forensic Science International. 197 (1–3): e1–e5. doi:10.1016/j.forsciint.2009.11.017. PMID 20018470.
- ^ a b Ingram AL (December 1964). "Morning Glory Seed Reaction". JAMA. 190 (13) (13 ed.): 1133–1134. doi:10.1001/jama.1964.03070260045019. PMID 14212309.
- ^ a b Kremer C, Paulke A, Wunder C, Toennes SW (January 2012). "Variable adverse effects in subjects after ingestion of equal doses of Argyreia nervosa seeds". Forensic Science International. 214 (1–3): e6–8. doi:10.1016/j.forsciint.2011.06.025. PMID 21803515.
- ^ a b Subramoniam A, Madhavachandran V, Ravi K, Anuja VS (December 2007). "Aphrodisiac property of the elephant creeper Argyreia nervosa". Journal of Endocrinology and Reproduction. 11 (2): 82–85.
- ^ a b c d e Marneros A, Gutmann P, Uhlmann F (October 2006). "Self-amputation of penis and tongue after use of Angel's Trumpet". European Archives of Psychiatry and Clinical Neuroscience. 256 (7): 458–9. doi:10.1007/s00406-006-0666-2. PMID 16783491. S2CID 9261722.
- ^ Chao JM, Der Marderosian AH (April 1973). "Ergoline alkaloidal constituents of Hawaiian baby wood rose, Argyreia nervosa (Burm. f.) Bojer". Journal of Pharmaceutical Sciences. 62 (4): 588–591. doi:10.1002/jps.2600620409. PMID 4698977.
- ^ a b c Paulke A, Kremer C, Wunder C, Achenbach J, Djahanschiri B, Elias A, et al. (July 2013). "Argyreia nervosa (Burm. f.): receptor profiling of lysergic acid amide and other potential psychedelic LSD-like compounds by computational and binding assay approaches". Journal of Ethnopharmacology. 148 (2): 492–497. doi:10.1016/j.jep.2013.04.044. PMID 23665164.
- ^ Wacker D, Wang S, McCorvy JD, Betz RM, Venkatakrishnan AJ, Levit A, et al. (January 2017). "Crystal Structure of an LSD-Bound Human Serotonin Receptor". Cell. 168 (3): 377–389.e12. doi:10.1016/j.cell.2016.12.033. PMC 5289311. PMID 28129538. See “LSD Diethylamide Stereoselectivity and Function”, figure 3, and “Discussion” (pages 382, 381, and 385). “Two noteworthy observations stand out. First, the key amide side chain of LSD—the group that distinguishes it from the far less hallucinogenic lysergamide (LSA)—adopts a constrained conformation in the binding site that cannot exchange readily with alternative conformational states.” (Discussion)
- ^ Halberstadt AL, Nichols DE (2020). "Serotonin and serotonin receptors in hallucinogen action". Handbook of the Behavioral Neurobiology of Serotonin. Handbook of Behavioral Neuroscience. Vol. 31. pp. 843–863. doi:10.1016/B978-0-444-64125-0.00043-8. ISBN 9780444641250. ISSN 1569-7339.
- ^ a b c d e f Gerhards N, Neubauer L, Tudzynski P, Li SM (December 2014). "Biosynthetic pathways of ergot alkaloids". Toxins. 6 (12): 3281–3295. doi:10.3390/toxins6123281. PMC 4280535. PMID 25513893.
- ^ a b c Willingale J, Atwell SM, Mantle PG (1983-07-01). "Unusual Ergot Alkaloid Biosynthesis in Sclerotia of a Claviceps purpurea Mutant". Microbiology. 129 (7): 2109–2115. doi:10.1099/00221287-129-7-2109. ISSN 1350-0872.
- ^ "Initial schedules of controlled substances (Schedule III), Section 812". www.deadiversion.usdoj.gov. Archived from the original on 2021-11-04. Retrieved 2020-01-17.
- ^ "Erowid LSA Vault : Legal Status". erowid.org. Retrieved 2020-05-05.
Further reading
[edit]- Powell W (2002). The Anarchist Cookbook. Ozark Press. p. 44. ISBN 978-0-8488-1130-3.
- Sydney S, Timmis GM (1932). "98. The Alkaloids of Ergot. Part III. Ergine, a New Base obtained by the Degradation of Ergotoxine and Ergotinine". J. Chem. Soc. 1932: 763–766. doi:10.1039/JR9320000763.
- Juszczak GR, Swiergiel AH (2013-01-01). "Recreational use of D-lysergamide from the seeds of Argyreia nervosa, Ipomoea tricolor, Ipomoea violacea, and Ipomoea purpurea in Poland". Journal of Psychoactive Drugs. 45 (1): 79–93. doi:10.1080/02791072.2013.763570. PMID 23662334. S2CID 22086799.
- Burillo-Putze G, López Briz E, Climent Díaz B, Munné Mas P, Nogue Xarau S, Pinillos MA, et al. (2013-09-01). "[Emergent drugs (III): hallucinogenic plants and mushrooms]". Anales del Sistema Sanitario de Navarra. 36 (3): 505–518. doi:10.4321/s1137-66272013000300015. PMID 24406363.
